By
Christian Lozeau, Bryan Hill, and Eric Bushnell
February 2023
Print Version
What you need to know
The carbonic anhydrase enzyme converts carbon dioxide and water into carbonic acid. If it is malfunctioning, it can cause numerous conditions, including glaucoma and epilepsy. With limited treatments available, we investigated novel therapeutics using a combination of computational and synthetic chemistry, resulting in promising leads.
Why this research is important
The carbonic anhydrase enzyme is found in many cells in the body, including blood, and is fundamental to breathing—something we all need to do to live. As different forms of this enzyme are located in many different areas of the body, it has been difficult to develop selective inhibitors for one specific action. For example, to prevent glaucoma without adversely affecting other body functions such as the liver or kidney. Developing selective inhibitors of carbonic anhydrase would allow for possible treatments without significant side effects elsewhere.
How this research was conducted
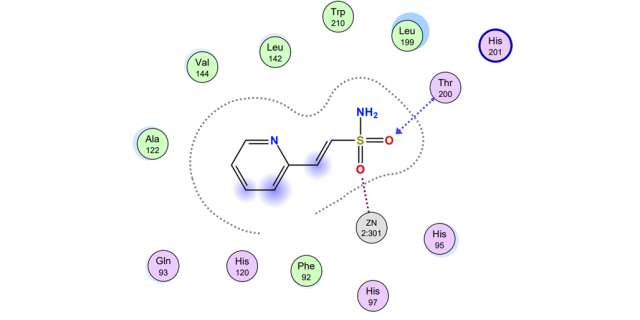
The research was a combination of computational chemistry and synthetic organic chemistry. Computationally, the carbonic anhydrase enzyme was loaded into the Molecular Operating Environment (MOE) software package. Several small novel sulfonamide-based inhibitors (i.e., drugs) were “docked” within the active site using MOE. The binding energies were then calculated between each of the inhibitors and the active site zinc ion and surrounding amino-acid residues. By comparing the binding energy of the various inhibitors, we could predict the effectiveness of each compound as a potential drug. In addition to the novel inhibitors, two known inhibitors, one a prescription drug (i.e., acetazolamide), were docked in the active site to generate a baseline binding energy for comparison. Experimentally, the next step is synthesizing the best-scoring inhibitors using a new methodology developed in the lab.
What the researchers found
Three of the four proposed novel inhibitors showed a stronger binding energy to the active site than the two known inhibitors and, therefore, legitimate synthetic targets. One of the target inhibitors is nearly synthesized in the lab requiring one final step to be complete, thus validating the new synthetic strategy.
How this research can be used
The novel inhibitors need to be tested biologically to determine their selectivity and efficacy as the next step. With that information, potential therapeutics could be developed, and this approach could be applied to other biochemical systems.
Acknowledgements
Eric Bushnell thanks NSERC for funding. Bryan Hill thanks the Dean of Science for funding associated with this project. Christian Lozeau thanks the Manitoba Métis Federation for their financial support.
About the Researchers
Keywords
- carbonic anhydrase
- computational chemistry
- MOE
- sulfonamides
Publications Based on the Research
Lozeau, C. (2022, June 13-17). Insights into the design of human carbonic anhydrase inhibitors. [Poster presentation]. 105th Canadian Society for Chemistry Conference and Exhibition 2022, Calgary, Canada. https://www.xcdsystem.com/cic/program/bMlXk1m/index.cfm?pgid=2690&sid=24559&abid=90988
Editor: Christiane Ramsey
Read more BU Research
Research at Brandon University follows comprehensive policies designed to safeguard ethics, to ensure academic integrity, to protect human and animal welfare and to prevent conflicts of interest.



